The effects of various components and other process parameters in the Zn-Fe alloy plating solution on the iron content in the coating were investigated. Under the optimal Zn-Fe alloy plating process, a Zn-Fe alloy coating with 0.41% iron content was obtained. The corrosion resistance of the alloy coating after silver-white passivation was about the same thickness as pure zinc coating. 2~3 times. The research also shows that the Zn-Fe alloy plating bath has good dispersing ability, covering ability and leveling ability, high electroplating current efficiency and excellent plating performance.
Since the 1980s, developed industrial countries have carried out extensive research on Zn-Fe alloy plating on the basis of pure zinc plating, especially in Japan and Europe, some manufacturers apply Zn-Fe alloy plating technology to industrial surfaces. Dispose of to meet the needs of vehicles in coastal areas, the salt sands of the Middle East, North West Europe and Canada that need to be dissolved in snow and salt. The Zn-Fe alloy coating is the same as the pure zinc coating. It is used as the anode protective coating for steel. It plays a mechanical and electrochemical protection role. This paper studies the Zn-Fe alloy plating process.

1 experimental research method
1.1 Test equipment and electroplating process
A 100 mL beaker was used as the plating bath, the cathode was a copper plate, and the anode was a zinc plate. The JW2 type DC source supplies current. The pH is measured with a pH meter. The iron content of the coating was measured by a 721 spectrophotometer [3]. The plating solution is prepared with a chemically pure reagent.
The electroplating process is: polishing of the plated parts → washing of water → chemical degreasing (10~15g/L Na2CO3 + a small amount of OP-10) → water washing → chemical etching → HCl 20% ~ 80% (volume ratio) → water washing → electrodeposition Zn-Fe Alloy→washing→lighting→silver white passivation→empty stop→washing→dry aging
1.2 plating composition and process conditions
Zinc chloride concentration / gL-1 60~90
Ferrous chloride concentration / gL-1 4~8
Potassium chloride concentration / gL-1 130~180
Sodium citrate concentration / gL-1 7~17
Boric acid concentration / gL-1 10~20
Additive concentration / gL-1 45~60
Dk/A.dm-2 1~2
pH 4.0~4.8
T/°C 20~30
Ascorbic acid concentration / gL-1 101.3 Test methods for plating solution and coating properties
The performance of Zn-Fe alloy plating bath plays a crucial role in the quality of the coating. Among all the properties of the electrolyte, the most important is its dispersing ability (ratio plating ability) and covering ability (deep plating ability). The author uses the far-near cathode method (Haring method) to measure the dispersing ability of ZnFe alloy plating solution. The calculation formula used is:
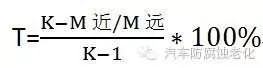
In the middle
T represents the throwing ability;
K is the ratio of the distance between the cathode and the anode;
M near near cathode weight gain, g;
M far cathode weight gain, g.The coverage of the Zn-Fe alloy plating bath was measured by the internal hole method. A copper tube of ɸ10 ​​mm 100 mm was used for the test, and the exterior was varnish-insulated. The tubular cathode is perpendicular to the anode during the measurement. After the test, the copper tube was longitudinally cut and the length of the plating in the hole was measured. The cathode current efficiency ŋ was measured by the weight measurement method.
The leveling performance of the Zn-Fe alloy plating bath is measured by HullCell. During the test, the center of the test piece will be polished to the surface of the specular bright film with a knives for lateral scratching. The disappearance of scratches before and after electrodeposition is observed, and the electrolyte leveling is qualitatively judged. The pros and cons of performance. The internal stress of the coating was measured by the HullCell test. On the cathode sheet of HullCell, eight vertical nicks (scoring width <0.1 cm) with a pitch of about 1 cm were placed on the cathode sheet, and the back surface was varnish-insulated. After plating for a certain period of time, take it out and observe the bending of the plate. The plating adhesion was tested by a scribe line. During the test, two sets of cross-parallel lines with a distance of 2 mm were scored on the surface of the coating with a knives with a cutting edge of 30°. Each line was drawn to the metal substrate to observe whether the plating at the intersection was peeled off. The evaluation of the appearance of the coating was tested by the HullCell. The HullCell plate was polished by 07# water sandpaper before the test, and the back of the plated (Cu) was varnished. After the test, 10 judges scored the brightness and brightness range of the observed HullCell plate, and then used C language programming to fuzzyly evaluate its brightness and its brightness range.
2 experimental results and discussion
2.1 Influence of process parameters on the iron content of the coating
2.1.1 Effect of Fe2+/Zn2+ ratio on coating iron content When the co-deposition ion concentration ([Zn2+]+[Fe2+]) was 0.6806mol/L, the effect of Fe2+/Zn2+ ratio (molar ratio) on the iron content of the coating was investigated. The test results are shown in Figure 1. When the Fe2+/Zn2+ ratio is increased, the iron content of the coating increases, and the abnormal co-deposition law is observed. Figure 1 also shows that the Fe2+/Zn2+ ratio is 0.076~0.10, and the iron content of the Zn-Fe alloy coating is in the optimum range of 03%~05%.
Relationship between Fe2+/Zn2+ ratio and Fe2+/Zn2+ ratio and iron content in Fig.1
2.1.2 Relationship between total concentration of co-deposited ions and iron content of coating
Maintaining the Fe2+/Zn2+ ratio of 0.076, the relationship between the total concentration of co-deposited metal ions (Fe2+, Zn2+) (mol/L) and the iron content of the coating is obtained, as shown in Fig. 2. It can be seen from Fig. 2 that the total concentration of co-deposited ions is increased, and the content of metal zinc having a lower potential is increased in the plating layer, showing abnormal co-deposition.
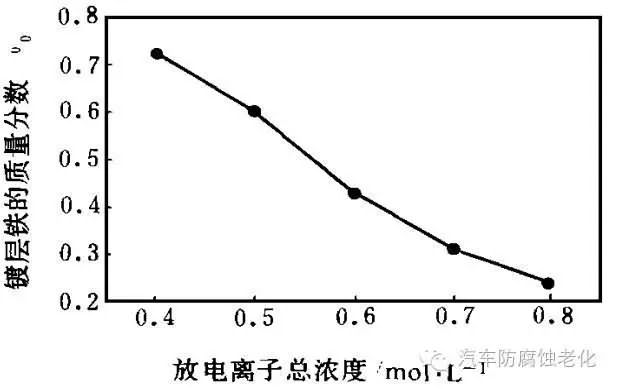
Figure 2 shows the relationship between the total concentration and the iron content of the coating
It was observed that when the concentration of co-deposited metal ions in the plating solution was low, the coating layer was rough and burr-like; the total concentration was increased, the burrs were reduced, and the plating layer was bright and flat. It indicates that the electrodeposition is controlled by concentration polarization when the concentration is low. Figure 2 also shows that the total concentration of co-deposited ions is preferably controlled at 0.5-0.7 mol/L.
2.1.3 Effect of potassium chloride concentration on iron content of coating
During the test, the Fe2+/Zn2+ ratio was 0076, and the total concentration of co-deposited metal ions was 068 mol/L. The relationship between the KCl concentration and the iron content of the coating was obtained, as shown in Fig. 3. It can be seen from Fig. 3 that as the KCl concentration increases, the iron content of the coating increases. This is because the chloride ion forms a complex ion with the zinc ion, which leads to an increase in the precipitation potential of the zinc ion, thereby facilitating the precipitation of iron. Changing the KCl concentration has little effect on the appearance of the coating.
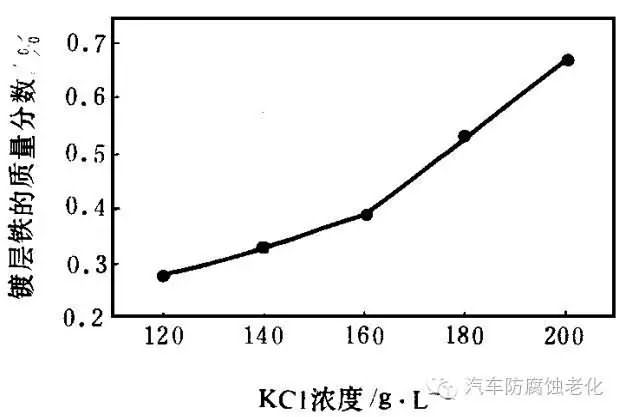
Figure 3 Effect of potassium chloride concentration and coating content
2.1.4 Effect of sodium citrate on the iron content of coating
The FeCl2 ratio is 6.2 g/L, and the relationship between the C6H5Na3O7/FeCl2 ratio (molar ratio) and the iron content of the coating layer is obtained, as shown in Fig. 4. It can be seen from Fig. 4 that as the content of C6H5Na3O7 in the plating solution increases, the iron content of the coating decreases first and then rises. This is because C6H5Na3O7 is not only a leveling agent but also a complexing agent. It can adsorb on the surface of the electrode and act like a surfactant. At this time, the inhibition of Fe2+ ion discharge by C6H5Na3O7 is much greater than that of Zn2+ ion discharge, resulting in an increase in iron content in the coating. And falling. At higher concentrations, C6H5Na3O7 can also complex with Zn2+, increasing the cathodic polarization of Zn2+ and increasing the iron content of the coating.
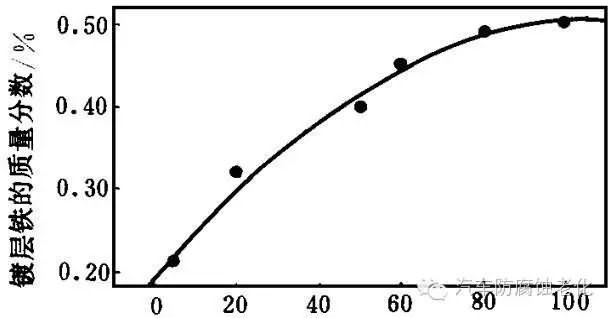
C6H5Na3O7/FeCl2 ratio (molar ratio)
Figure 4 Relationship between sodium citrate and iron contentSodium citrate is allowed to have a wide concentration range and a molar ratio of 0.5 to 1.2. However, when the concentration of citric acid is more than 17 g/L, the coating layer is rough, burr-like, and a zinc citrate precipitate is formed.
2.1.5 Effect of additives on the iron content of the coating
The additive has a significant influence on the iron content of the coating. As can be seen from Fig. 5, when the amount of the additive is increased, the iron content of the coating is increased, and when the amount of the additive exceeds 80 ml/L, the iron content of the coating reaches 0.5% and remains substantially unchanged.
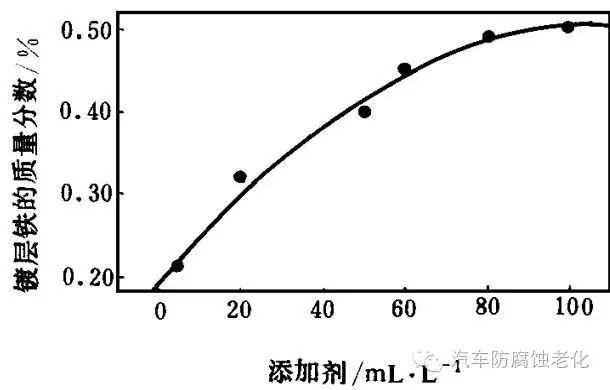
Figure 5 Relationship between additives and iron content
2.1.6 Effect of pH on the iron content of the coating
The effect of pH on the iron content of the coating is complex (Figure 6). It can be seen from Fig. 6 that the iron content of the coating increases with the increase of the pH value; when the pH exceeds 5.0%, the iron content of the coating decreases. This is because when pH is low, Fe2+ forms an acid complex with citrate Fe. HCit (lgβ=19.1) and [FeH2Cit]+ (lgβ=24.2), and their cumulative stability constants are large, so the iron content is low; When the pH is increased, the complex gradually changes to [FeCit]-(lgβ=15.5), and the cumulative stability constant is small, so the iron content increases; after the pH reaches 5.0, the Fe2+ and citrate side reaction coefficient (lgαM(L) =4.2) is greater than the Zn2+ and citrate side reaction coefficient (lgαM(L) = 3.2), and therefore, the iron content of the coating is lowered. The pH should be controlled between 4.0 and 4.8.
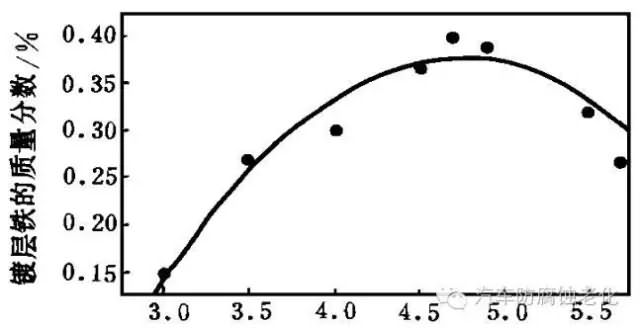
pH
Figure 6 Effect of pH on the iron content of the coating2.1.7 Effect of current density on coating iron content
The relationship between current density and coating iron content is shown in Figure 7. As the current density increases, the coating iron content increases. When the current density is less than 1A/dm2, the iron content is linear with the current density; when it is greater than 1A.dm2, the iron content increases slowly; after reaching 1.5A/dm2, the iron content is close to a constant value. This is because the 4s valence orbital level of Fe2+ in the plating solution is higher than the 4s valence orbital level of Zn2+, and as the electrode current density increases, the Fermi level of the electrode increases, which is beneficial to the electrodeposition of Fe. The iron content in the coating increases. It has been reported in the literature that when the concentration of Cl- in the electrolyte is less than 305g/L, the content of Fe in the Zn-Fe alloy increases with the increase of current density. The literature reports are in good agreement with the experimental results. It can be seen from Fig. 7 that the current density is controlled in the range of 1.0 to 2.0 A/dm2, and a Zn-Fe alloy having an iron content of 0.3% to 0.5% can be obtained.
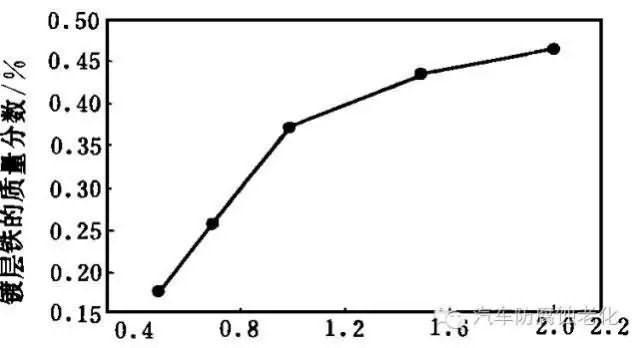
Current density / A.dm-2
Figure 7 shows the relationship between current density and iron content.2.1.8 Effect of temperature on the iron content of the coating
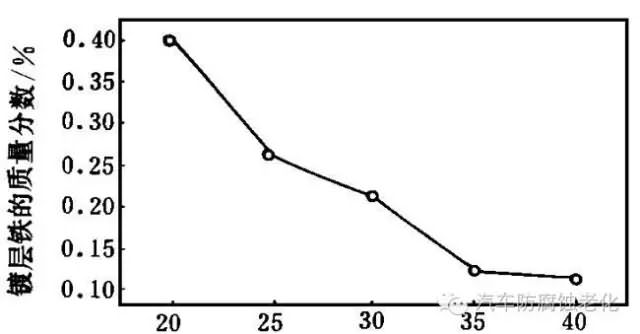
The effect of temperature on the iron content of the coating is more pronounced (Fig. 8). As can be seen from Figure 8, the iron content of the coating decreases with increasing temperature. This is because the activation energy of Zn2+ electrochemical reaction is ΔHZn*>ΔHFe*. Therefore, as the temperature of the plating solution increases, the mass fraction of iron in the coating decreases. Temperature control at 20~30 °C can obtain alloy plating with iron content of 0.3%~0.5%.
Temperature / °C
Figure 8 Relationship between temperature and coating iron content2.2 Zn-Fe alloy plating solution performance test results
2.2.1 Corrosion resistance of Zn-Fe alloy coating
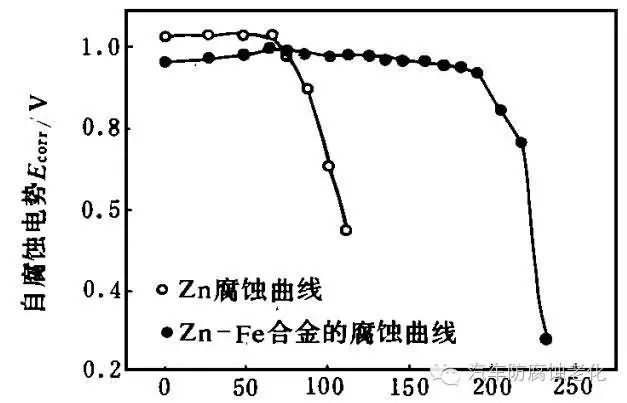
The optimum conditions for experimental control were: ZnCl286g/L, FeCl26.2g/L, KCl160g/L, C6H5Na3O712g/L, H3BO316g/L, additive 45mL/L, pH=4.6, DK=1A/dm2, temperature 20°C. A Zn-Fe alloy plating layer having an iron content of 0.41% and a thickness of 7.6 μm and having a smooth brightness was obtained. The coating was inactivated in the silver-white passivation solution for 4 min simultaneously with the pure zinc coating of the same thickness, and then immersed in a 5% NaCl solution to determine the relationship between the corrosion potential Ecorr and the time t. Figure 9. It can be seen from Fig. 9 that the corrosion resistance of the ZnFe alloy is about 2 to 3 times that of the pure zinc coating.
Time / h
Figure 9 Ecorrt curve of Zn-Fe alloyAfter the introduction of the iron-group transition metal in the ordinary zinc coating, the corrosion resistance of the formed Zn-Fe alloy is much higher than that of the ordinary zinc coating, and the reasons are various: First, it can be seen from FIG. The stable potential of the Zn-Fe alloy is positive than that of zinc, but negativer than iron. Therefore, the ZnFe alloy coating, like the ordinary zinc coating, is an anodic protective coating relative to the base steel, which not only has electrochemical protection, but also does not cause excessive corrosion of the coating due to excessive electromotive force of the microbattery. According to the literature, the surface of the Zn-Fe alloy coating has tiny cracks, which can disperse the corrosion current and improve the protection performance. Secondly, the grain size of the passivated Zn-Fe alloy coating is more uniform than that of the pure Zn coating, and the preferred orientation of the crystal is (101) plane, and a small amount of iron in the coating migrates into the structure of the passivation film, and chromium. The acid forms a relatively stable hydrophobic compound, which greatly improves the corrosion resistance of the ZnFe alloy coating. On the other hand, a small amount of iron in the plating layer can also increase the content of Cr2O3 and CrO3 having high corrosion resistance in the passivation film of the alloy plating layer. Among them, CrO3 is the main component of corrosion resistance. It has an automatic repairing effect on the passivation film. When the passivation film is partially destroyed, it can interact with zinc in the coating to form a new passivation film. The higher the content of CrO3, the more obvious the repairing effect and the better the corrosion resistance. Cr2O3 in the passivation film can firmly passivate the film to make it bond well with the substrate. It can also be seen from Fig. 9 that in the etching process of the Zn-Fe alloy, since the stable potential of the Zn-Fe alloy is positive than zinc, it is more negative than iron. Therefore, when Zn in the Zn-Fe alloy begins to corrode to form a Zn(OH)2 colloid, a small amount of iron in the coating remains unetched, and its presence causes a surface of the ZnFe alloy coating to form a surface more than the surface of the zinc coating. The granules with large granules are formed, and each of the adjacent spherulites leaves a skeleton which is difficult to corrode, and this skeleton is filled with the corrosion product Zn(OH)2, thereby suppressing corrosion.
2.2.2. Plating solution and coating performance test results
2.2.2.1 Dispersive ability. When the ratio of the distance between the cathode and the anode is K=2, the dispersion capacity of the plating solution is T=65%.
2.2.2.2 Covering ability of plating solution. When the coverage of the Zn-Fe alloy plating solution was measured by the internal hole method, the length of the inner plating of the copper tube was 69 mm.
2.2.2.3 Cathodic current efficiency of the bath. The cathode current efficiency of the plating solution was measured by the weight measurement method ŋ ≥ 93%.
2.2.2.4 The flattening ability of the plating solution, the HullCell test piece with lateral nicks was deposited in the Zn-Fe alloy plating solution for 5 minutes, and the trace disappeared completely, indicating that the leveling performance of the plating solution was good.2.2.2.5 Internal stress of the coating. After 5 minutes of electrodeposition, the HullCell test piece with vertical scribe lines showed almost no bending deformation, indicating that the internal stress of the coating was small.
2.2.2.6 Bonding strength of the coating. After the plating was cross-hatched, no plating peeled off at the intersection of the scribe lines, and the plating between the scribe lines did not peel off. It shows that the bond between the coating and the substrate is firm and the bonding force is strong.
2.2.2.7 Appearance of the coating. When the current intensity is 0.5A, the surface of the HullCell test piece is flat and fine, and all bright. Using the formula, it can be seen that a smooth and dense surface of the bright coating can be obtained in a wide current density range of DK=0.732~4.686A.dm2.
3 conclusions
3.1 The process of electrodeposition of Zn-Fe alloy by weakly acidic chloride was determined, and the important functions of each component were discussed. The optimum process bars of Zn-Fe alloy were: ZnCl286g/L, FeCl262g/L, KCl160g/L, C6H5Na3O712g/L, H3BO316g/L, additive 45mL/L, pH=4.6, DK=1A/dm2, temperature 20°C. In the optimum process conditions of Zn-Fe alloy, a Zn-Fe alloy coating with an iron content of 0.41% and a thickness of 7.6 μm was obtained. The coating was simultaneously passivated with pure zinc coating of the same thickness for 4 min, and then immersed in 5% NaCl solution. The results show that the corrosion resistance of Zn-Fe alloy is about 2 to 3 times that of pure zinc coating.
3.2 The reason why Zn-Fe alloy has high corrosion resistance is analyzed. It is considered that its high corrosion resistance is determined by the structure of zinc-iron alloy itself.
3.3 The properties of the electrodeposited Zn-Fe alloy plating bath and the properties of the ZnFe alloy coating obtained therefrom were determined. The results show that the bath has good dispersing ability and covering ability, strong leveling ability, stable and reliable plating system; the obtained ZnFe alloy coating has small internal stress and strong bonding force with the substrate, and the surface of the coating is smooth and silvery. And the coating has excellent corrosion resistance.
Floor-Stand Digital Signage,Wayfinding Information Kiosk,Media Ads Player,Lcd/Led Advertising Display
Shenzhen Sunson Tech Co., Ltd , https://www.sunsonkiosk.com