Under the constant stimulation of cruising range anxiety, liquid lithium metal batteries have become a focus of attention due to their high energy density, but the serious failure of lithium metal negative electrodes has restricted the commercial development of lithium metal batteries. At present, there are still controversies in the academic circles about the mechanism of lithium metal anode failure and protection. The traditional view is that the growth of lithium dendrites is the main reason for the failure of lithium metal anodes. However, in fact, although the metal lithium negative electrode without dendritic growth is reported in the literature, the practical lithium metal battery using high areal capacity positive electrode (≥ 2 mAh cm-2) and ultra-thin lithium negative electrode (such as 50 μm) is usually Capacity diving failure will occur within 100 charge and discharge cycles, which is far inferior to the cycle performance of lithium-ion batteries under the same capacity. When disassembling a practical lithium metal soft pack battery whose capacity has failed to dive, severe powdering of the metal lithium negative electrode is usually observed. But at present, the academic circles are still not clear about the origin and composition of the powdering of lithium metal anode.
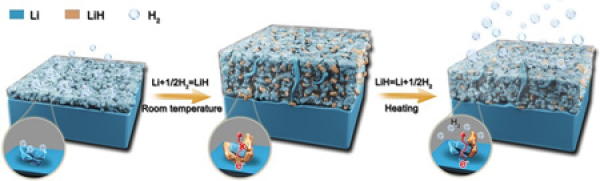
In recent years, Cui Guanglei and Dong Shan, researchers at the Solid State Energy System Technology Center of Qingdao Institute of Bioenergy and Processes, Chinese Academy of Sciences, have made a series of research results in the fields of lithium metal batteries and lithium metal anode protection (Chem. Mater. 2017, 29, 4682- 4689; Chem. Sci. 2018, 9, 3451-3458; Chem. Mater. 2018, 30, 12, 4039-4047; Angew. Chem. 2019, 131, 5997-6001; Small 2019, 15, 1900269; Chem. Mater . 2020, 32, 8, 3405-3413). In the process of advancing these work, the researchers found through online differential electrochemical mass spectrometry that a large amount of H2 is produced in the lithium metal battery during the charge and discharge process. Will the lithium metal negative electrode react with H2 to form LiH? Will LiH be the culprit for the expansion and powdering failure of the lithium metal anode? The researchers upgraded the online differential electrochemical mass spectrometry system to realize the online titration gas analysis function. The lithium metal anode was titrated with deuterium water (D2O) (criterion: 2Li + 2D2O → 2LiOD + D2↑; LiH + D2O → LiOD + HD↑). This study found for the first time that there are a lot of poor conductivity in the failed powdered lithium metal anode Lithium hydride (LiH); the cycle performance of practical lithium metal batteries (2.805 mAh cm-2 LiCoO2, 50 μm Li) is negatively correlated with the accumulation of LiH in the lithium metal anode. The study revealed that the formation and decomposition of LiH is determined by a temperature-sensitive chemical equilibrium (Li + 1/2H2 LiH): at room temperature, H2 generated by the interface side reaction reacts with lithium metal to form LiH; LiH will be partially decomposed by heating Produces lithium metal with excellent conductivity and electrochemical activity, thereby recovering and increasing capacity. Studies have shown that effectively inhibiting the generation of H2 and the accumulation of LiH is of great significance to the protection of lithium metal negative electrodes, which provides a new idea for the development of practical lithium metal batteries: on the positive electrode side, the electrolyte oxidation product R-H+ shuttles to the negative electrode Reduction is the main reason for the generation of H2. The two strategies of passivating the positive electrode and preparing an electrolyte with less hydrogen are used to inhibit the generation of R-H+; the functional treatment of the separator or polymer electrolyte prevents R-H+ from shuttle to the negative electrode; Lithium anodes construct interface protection materials with strong hydrogen storage or hydrogen absorption capabilities. In fact, the currently reported interface components (such as LiF, Li3N, BN, Li2O and nano-carbon materials) that can effectively protect metal lithium anodes are excellent Hydrogen storage material; adopts heating and pressurization strategy. In addition, the researchers suggested that the characterization of metal hydrides on the electrode interface should be strengthened in various battery systems, which will create a new direction for battery interface research.
Recently, related research results were published on Angewandte Chemie International Edition. Xu Gaojie, Li Jiedong, and Wang Chao, teachers of the Solid State Energy System Technology Center of Qingdao Energy Institute, are the co-first authors of the paper, and Cui Guanglei and Dong Shanshan are the corresponding authors. The research work is supported by the National Key R&D Program, the Chinese Academy of Sciences Strategic Pilot Science and Technology Project, the National Science Fund for Outstanding Youth, the Chinese Academy of Sciences Youth Innovation Promotion Association, and the Shandong Provincial Key R&D Program.
Best Kitchen Gadgets,New Kitchen Gadgets,Chef Tools,Kitchen Utilities
Garwin Enterprise Co.,Ltd , https://www.garwincn.com