Since the aliphatic polyamide contains an amine group and a carbonyl group, it easily forms a hydrogen bond with water molecules, so that various materials obtained are easily absorbed during use, and a plasticizing effect occurs, resulting in volume expansion of the material and a decrease in modulus under stress. Significant creep has occurred.
Polycaprolactam and polyhexamethylene adipamide (nylon 6 and nylon 66) are the most commonly used polyamide materials . They absorb up to 10% by weight of moisture from humid air and absorb mass fraction under normal humidity conditions. 2% to 4% moisture, resulting in a variety of mechanical properties.

Nylon 6 and nylon 66 are very different in the scope of this discussion, collectively referred to as nylon 6/66. This paper summarizes the research on the mechanism of water absorption of nylon 6/66 and improving its hygroscopicity.
(1) Effect of moisture on the properties of nylon 6/66
After nylon 6/66 absorbs water, various properties change, and many changes in properties are related to the amount of water absorbed.
1. Crystallinity and crystal structure
The crystallographic study of nylon 6/66 found that nylon 6/66 is a semi-crystalline material, which contains both crystalline and amorphous regions after molding. In the crystal region, the molecular chain is in a planar sawtooth conformation, and a hydrogen bond is formed between the chain and the chain through an amide bond. In the amorphous region, the molecular chain conformation is random, and most of the amide bonds do not interact to form a hydrogen bond, which is in a "free" state, but does not exclude the formation of local hydrogen bonds in a few regions.
In earlier studies, nylon crystallinity was often estimated by density. The density of nylon 6/66 is larger than that of water. After water absorption, the density of these two materials increases and the crystallinity also rises. The stretch oriented nylon 6/66 material often contains a portion of the gamma-crystals. It has been found that the γ-crystal ratio of the nylon material after water absorption is reduced, and the more stable α-crystal ratio is increased.
2. Mechanical properties and molecular motion
The change in mechanical properties of nylon after water absorption is obvious. The most important are the decrease in hardness, modulus and tensile strength, decrease in yield point, and increase in impact strength.
The molecular motion of nylon 6/66 has nuclear magnetic resonance, dynamic mechanical relaxation and dielectric loss. The transformation of nylon 6/66 material before and after water absorption is studied. The glass transition temperature (Tg) is sensitive to moisture. Tg has fallen sharply. For example, when the water content of nylon 6 is 0.35% w/w, Tg=94° C., 10.33% w/w, Tg=-6° C; dry nylon 66 Tg=78° C., when the water content is 11% w/w, Tg=40 °C. At the same time, it was found that the process of decreasing Tg with increasing water absorption is phased. The initial decline is rapid; when the water absorption mass fraction exceeds a certain value, the decline is slow.
According to various literature reports, the critical value is about 2% to 4%. Nylon 6/66 also exhibits beta and gamma transitions at lower temperatures, where the beta transition is only observed in wet samples and its intensity increases with increasing water absorption. Some studies have also found that the increase in the intensity of the beta transition peak is accompanied by a decrease in the gamma transition peak and exhibits a phase similar to Tg.
All of the above phenomena indicate similar plasticizing effects. However, when the test temperature is further lowered, after a certain critical temperature, the effect of moisture in the nylon 6/66 material is reversed, similar to cross-linking hardening. The specific value of this critical temperature is quite different in different reports. It has been suggested that this is related to the difference between the dynamic mechanical test frequency and the degree of orientation of the sample.
Nylon will harden after being subjected to stresses less than the yield point for a long time. This effect is called "stress aging". After absorbing water, the rate of stress aging is accelerated.
3, size changes
After the nylon 6/66 absorbs water, the volume will expand. When expanding, material size changes and changes in water absorption are not completely synchronized. Nylon 6 fiber expands faster and slower with the change of water absorption; while nylon 6 film is the opposite. The sample having a stretched orientation has an anisotropy. The expansion is more pronounced in the direction of the stretch orientation.
It was found that nylon 6/66 under tension has an intermolecular hydrogen bond orientation which is close to the direction of stretching. Therefore, it is considered that the water swelling of nylon 6/66 is more obvious in the direction of intermolecular hydrogen bonding.
4, heat setting method
Nylon 6/66 fiber production has two methods of moist heat setting and dry heat setting. The study found that in the case of the same crystallinity, the dry heat setting sample has less water absorption than the damp heat setting. The wet heat setting sample has better dyeing performance.
(2) Mechanism of water absorption of nylon 6/66
Summarizing the previous research, it is basically believed that water molecules only enter the amorphous region of nylon 6/66, and the molecular chain activity increases after water absorption, which plays a role of plasticization. This is the cause of the crystal transformation, the decrease in Tg, and the occurrence of new relaxation mentioned in the previous section.
In view of the segmentation of the process of Tg and other properties changing with the increase of water absorption, Puffr and Å ebenda proposed the mechanism of nylon 6/66 step-by-step water absorption, which was supported by a large number of experimental results.
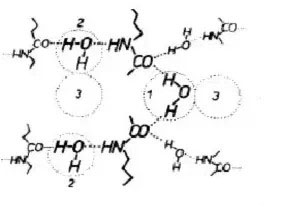
The mechanism believes that water molecules enter the amorphous region of nylon 6/66, which is preferentially bound in the form of 1 in the figure (tightly bound). When the water molecules continue to increase, the combination form shown in Figure 2 appears (loose) Combined, loosely bound, more water molecules will be further accumulated in the molecular gap by hydrogen bonding between water molecules (clustering, as shown in Figure 3).
The segmentation effect of nylon 6/66 mentioned in the previous section on dynamic mechanical relaxation, dielectric relaxation and stress aging with the change of water absorption is the embodiment of P-Å stepwise water absorption mechanism. The segmentation effect with the change of water absorption is also found in the properties of fatigue crack growth and fracture energy, which can be explained by the P-Å mechanism. At the same time, the broad-line NMR absorption spectrum and relaxation time also found that only a part of the water molecules absorbed by nylon 6/66 were mobile, indicating that they contain two types of water molecules with different degrees of binding. The positron annihilation lifetime spectrum study shows that the free volume of nylon decreases first and then increases with the increase of water absorption, which is also consistent with the P-Å mechanism.
The theoretical description of nylon water absorption can be described by the Flory-Huggins equation or the Zimm equation (Zimm equation is the development of the Flory-Huggins equation). The results of comparing these theories with the experimental results support the mechanism of P-Å two-step water absorption. In addition, this mechanism is also supported by molecular modeling methods.
(3) Method for solving the problem of nylon 6/66 water absorption
It can be known from the above conclusion that the plasticizing effect of water on nylon 6/66 material is obvious, and it is most sensitive in the initial water absorption stage. It is difficult to maintain the performance of nylon 6/66 materials by simply maintaining a dry environment. There are two ways to solve the problem of nylon 6/66 water absorption. One is to reduce the influence of moisture on its performance by reducing the water absorption. The other is to improve the performance of nylon 6/66 after water absorption by improving the performance of nylon 6/66. Impact.
1, blending and compounding
The addition of phenolic resin such as phenolic resin and polyvinylphenol can reduce the water absorption of nylon 6/66, increase its Tg, and have less influence on Tm.
The study found that the added phenolic substances are mainly found in the amorphous region of nylon 6/66. For the effect of phenolic substances, the researchers explained that water can destroy the hydrogen bonds that have formed in nylon 6/66 and form new hydrogen bonds with carbonyl or amine groups, because water molecules and carbonyl groups or The tendency of amine groups to form hydrogen bonds is higher than between them. The tendency of the phenol group to form a hydrogen bond with the carbonyl group is higher than that of the water molecule. After the addition of the phenolic substance, the phenol group occupies the carbonyl group and the amine group in the nylon 6/66, and the benzene ring contains a steric hindrance effect. The entry of water molecules is prevented. This interpretation is supported by isothermal adsorption experiments, SAXS and molecular simulation methods.
The addition of an amine polyether (Blox), a sulfonated polyester or an aramid-containing polyamide also reduces the amount of water absorbed by the nylon 6/66. The blending of nylon 6/66 with other polymers (such as PP, PS, PC, ABS, etc.) generally only slows down the water absorption rate and does not reduce the water absorption. At the same time, if the compatibility is not good, the mechanical properties will be sacrificed.
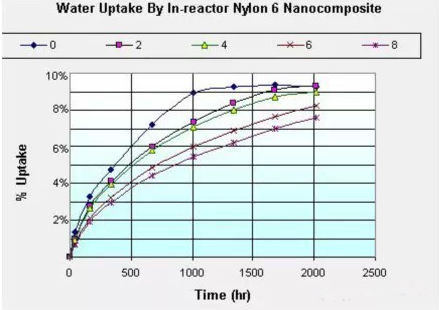
The addition of inorganic nanoparticles has a certain effect. Polyamide/layered silicate nanocomposites can greatly improve mechanical properties, heat distortion temperature, barrier properties and flame retardant effects, while organic montmorillonite polyamide/layered silicate nanocomposites can reduce water absorption. speed. When the addition of montmorillonite reaches a certain amount (usually greater than 4%), the equilibrium water absorption of nylon can be reduced. This is because montmorillonite acts as a nucleating agent to increase the crystallinity of the nylon, so that the amorphous region becomes smaller, thereby reducing the water absorption of the nylon.
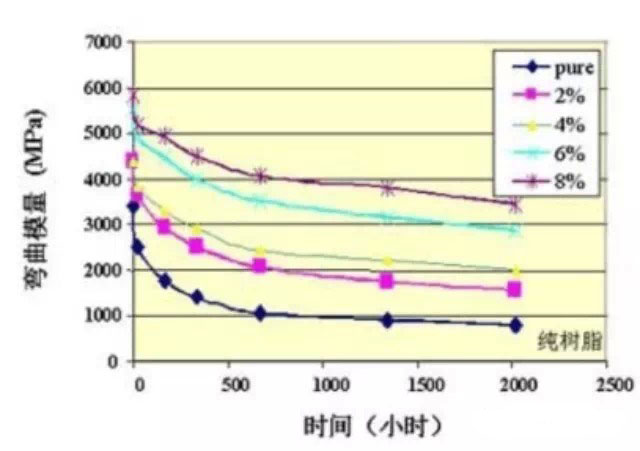
The above nanocomposite method can greatly improve the adverse effects of nylon 6/66 due to water absorption while slowing down the water absorption speed and reducing the water absorption. If the nylon montmorillonite nanocomposite absorbs water, the mechanical properties decrease and the dimensional change is smaller than that of pure nylon. On the other hand, the reports of these blends and inorganic nanocomposites cited above have been improved in mechanical properties, such as Tg, modulus increase, etc., even after water absorption, the mechanical properties have dropped, and the extent of fallback is much smaller. The increased amount after adding montmorillonite. The picture on the right is the nylon 6 material with different content of montmorillonite. After the period of time, the flexural modulus changes. It can be seen from the figure that even after the addition of 2% montmorillonite, after reaching the saturated water absorption rate, The flexural modulus will also be much higher than pure nylon 6.
It has also been reported that by bonding nylon 6/66 to a layered composite material having a relatively low hygroscopicity and high mechanical strength, the nylon 11 can maintain its size and a certain mechanical strength after water absorption due to the support or restriction of nylon 11.
2, cross-linking
The change in mechanical properties of nylon 6/66 after cross-linking is conventional, ie, Tg rise, rigidity and brittleness are enhanced. However, there have been few reports on the effect of water absorption or moisture on the properties of the material after cross-linking. Only one report has reported that the amount of water absorbed by nylon 6 after cross-linking has decreased.
3, surface modification
The amount of water absorption can be reduced by hydrophobizing the surface of the nylon 6/66 material. For example, a superhydrophobic layer having a lotus leaf structure is formed by surface grafting of a fluoropolymer or on the surface. The addition of an amine polyether (Blox), a sulfonated polyester or an aramid-containing polyamide also reduces the amount of water absorbed by the nylon 6/66.
Kn95 Fold Flat 3D Mask,Kn95 5 Layers Face Mask,Kn95 Fold Flat Respirator,Kn95 Filters Cup Face Mask
Jiangmen anjian biotechnology co. LTD , https://www.anjianmask.com